Get a glimpse into some of the fascinating and impactful research we’re working on in the Department of Surgery! Check out the winners and all submissions for our 2022 Surgery Science Image Contest, held in early January as part of our annual Research Summit.
First Place:
Smiling Bile from a Happy Liver
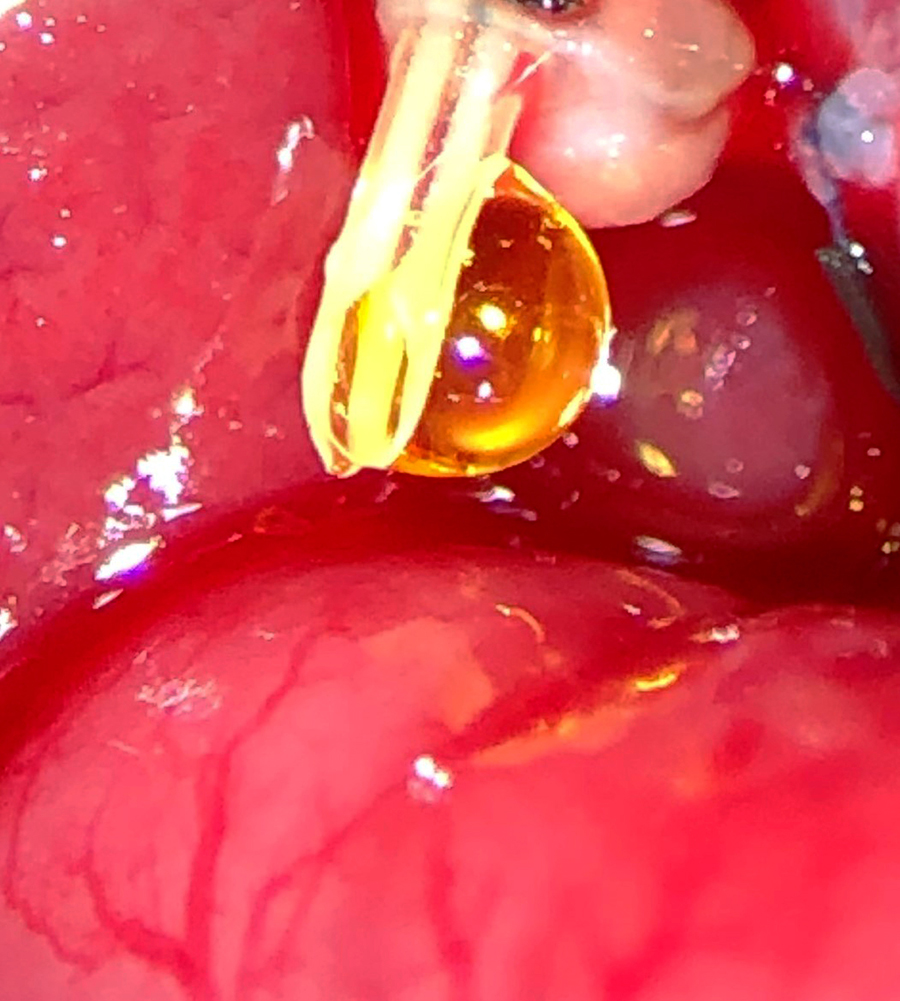
Weifeng Zeng, MD, Poore Lab; Bret Verhoven, Al-Adra Lab
We saw this smiling drop of bile during the first successful rat liver transplant at UW-Madison in more than two decades.
This surgery provides an excellent rodent liver transplant model for studying and improving human transplant outcomes.
Runner-up:
Nerve endings arborize near mechanoreceptor PIEZO2-expressing epithelia in P0 mouse larynx
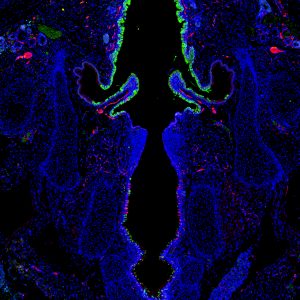
Alexander Foote, Graduate Student, Otolaryngology-Head & Neck Surgery
Section cut by Sierra Raglin; Immunostain performed by Alexander Foote; Susan Thibeault lab
This is a cross section of the larynx from a mouse pup day 0 after birth exhibiting the mechanoreceptor PIEZO2 (green), and innervating nerve fibers/neurons labeled via PGP9.5 (red). This image was taken with an inverted Nikon microscope in the Thibeault lab.
PIEZO proteins are cell-membrane receptors that are only expressed in mammalian cells. The proteins are activated when the cell stretches, and they convert these mechanical cues to biochemical cell responses. This image shows selective expression of PIEZO2 mechanoreceptor above and below the vocal folds in regions of the larynx where there is increased nerve supply (innervation). This suggests that PIEZO2-expressing cells in the larynx may directly or indirectly communicate with innervating sensory neurons that convey stimuli to the brain, resulting in airway-protective responses.
Runner-up:
Pain Management Counseling for the Surgical Patient
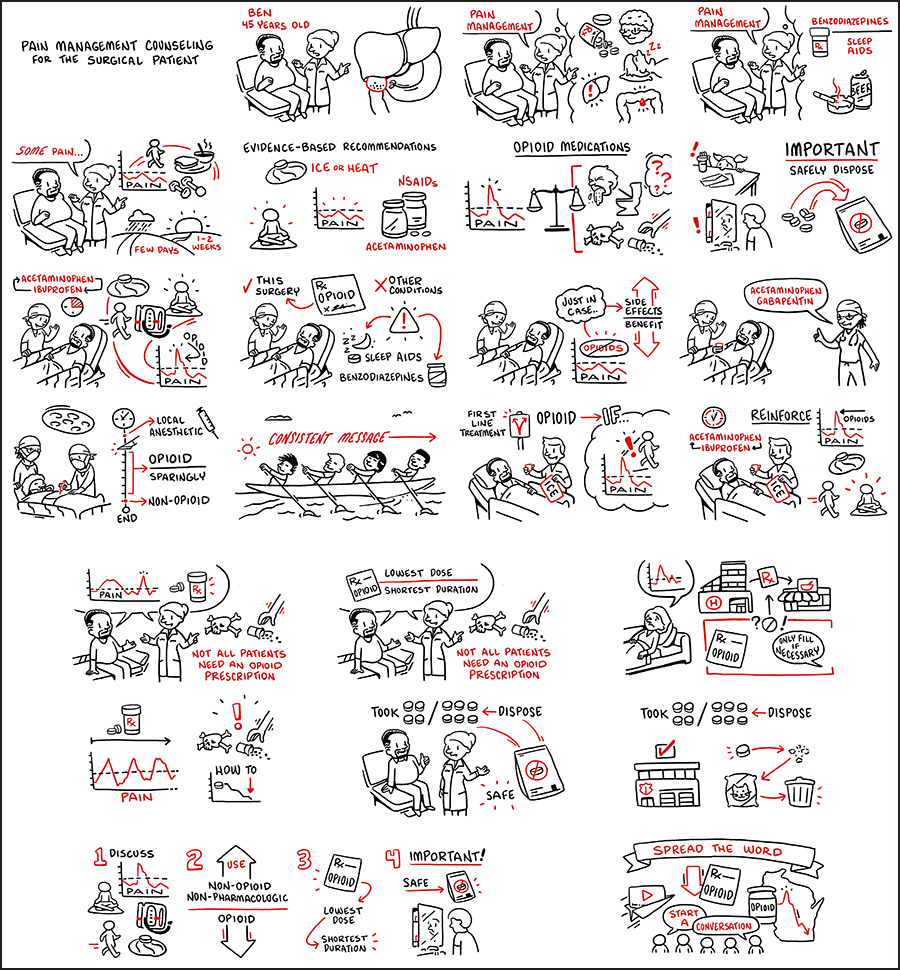
Sudha Pavuluri Quamme, MD, MS; Tudor Borza Lab
Surgical Collaborative of Wisconsin & TruScribe: Sudha Pavuluri Quamme, MD, MS; Joanne Peters, PhD; Tudor Borza, MD
This cartoon depicts a comprehensive approach to safe and effective management of surgical pain for a patient undergoing a laparoscopic cholecystectomy. It highlights a spectrum of pain management strategies to show surgical prescribers how to integrate these steps into their practice.
Hypoglossal motor neurons
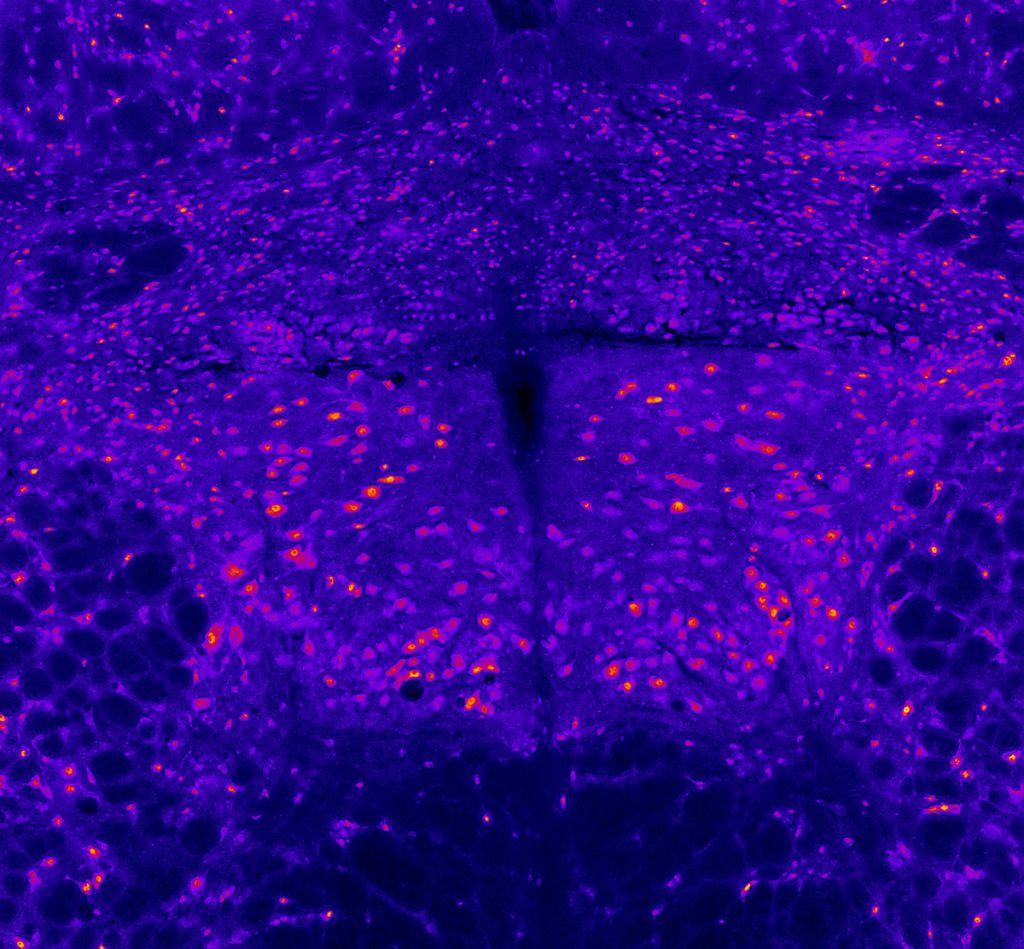
Miranda Cullins, PhD, Postdoctoral Fellow, Otolaryngology-Head & Neck Surgery; Nadine Connor Lab
This image depicts the hypoglossal motor neurons which activate the tongue muscles. This section of rat brainstem was imaged on a microscope using an immunofluorescent stain for neurons. The motor neurons appear as the larger pink-orange spots, clustered in the bilateral hypoglossal nuclei in the center of the image. Other smaller neurons appear in the surrounding tissue. This image was collected for a study designed to determine whether a loss of motor neurons contributes to weakness of the tongue after cerebral stroke.
Estrogen Receptors in the Vocal Fold
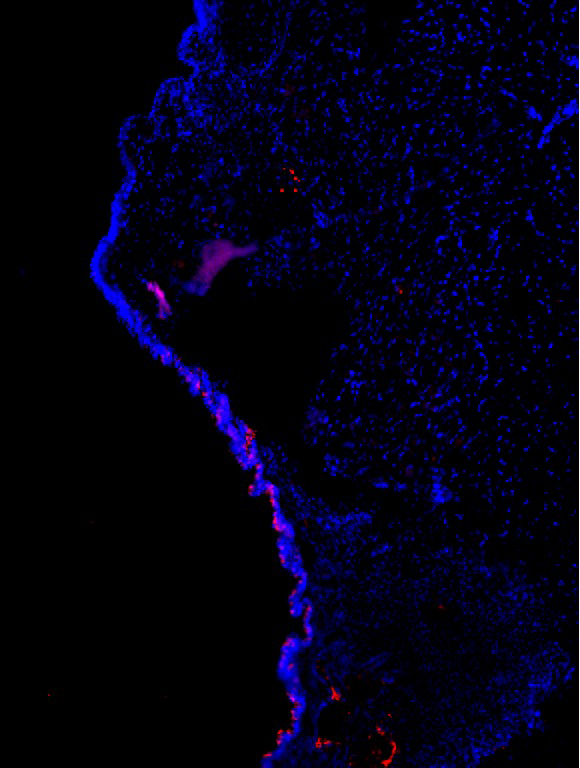
Charles Lenell, PhD, Postdoctoral Fellow, Otolaryngology-Head & Neck Surgery; Michelle R. Ciucci Lab
A cross-section of a right vocal fold (10 microns) of a Long-Evans rat. Blue labels the cell nuclei and red labels a specific estrogen receptor, GPER. GPER is present in the inferior portion of the epithelium of the vocal fold. This receptor is responsible for rapid estrogenic effects and could help explain why women’s voices are more susceptible to hormonal changes than male counterparts. Quantifying the type and ratio of hormone receptors in the vocal folds of both sexes is the first critical step to determining hormonal effects by defining receptors that potentially affect vocal fold biology.
Inflammatory Biomarker in the Vocal Folds of a Parkinson Rat Model
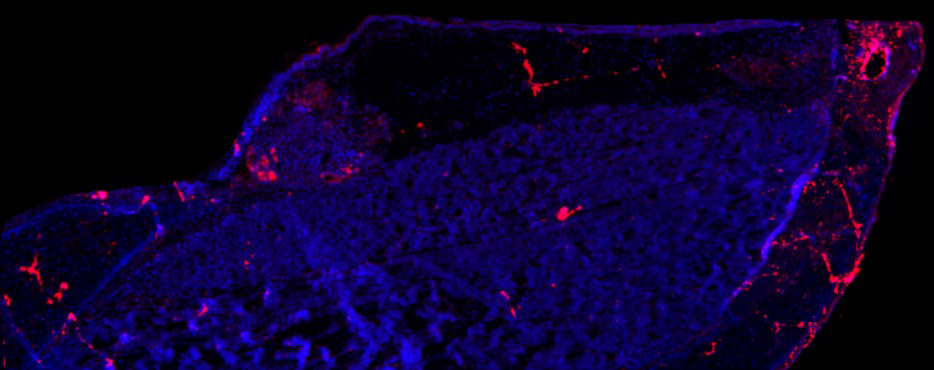
Charles Lenell, PhD, Postdoctoral Fellow, Otolaryngology-Head & Neck Surgery; Cynthia Kelm-Nelson Lab
A longitudinal section (4x) of a right vocal fold (10 micron) of a male rat with an early-onset Parkinson’s disease gene mutation (Pink1-/-). The image is stained for Caspase-7 (a protease involved in apoptosis and inflammation) in red and cell nuclei in blue. Increased expression of Caspase-7 in the vocal fold is associated with increased inflammation and cell apoptosis which may contribute negative changes in the vocal fold biology and functions associated with Parkinson’s disease. By understanding how the vocal folds are negatively impacted by Parkinson’s disease, future research can investigate how to prevent and reverse these changes.
Rat Thyroarytenoid Muscle Stained for Capillaries
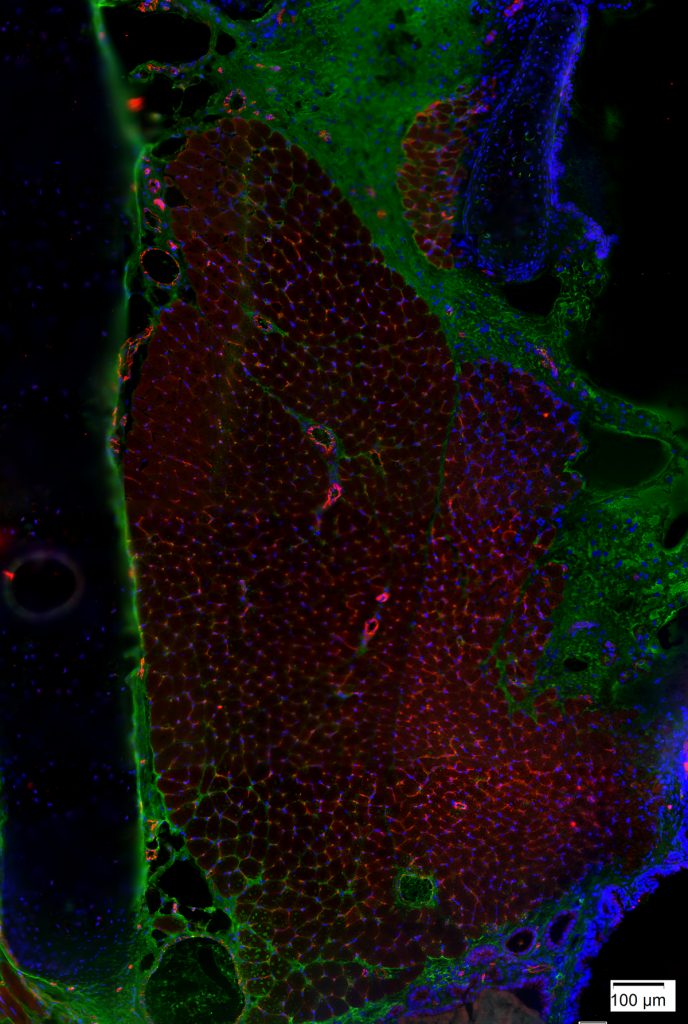
Jonathan Setzke and Tiffany Glass, PhD, MA, CMI; Nadine Connor Lab
This image depicts a section of rat thyroarytenoid (TA) muscle stained for capillaries with anti-CD31 (red) and nuclei with DAPI (blue). The thyroid cartilage is on the left and the airway is on the right. This tissue section was imaged using a light microscope at 20x magnification. The staining shows the density of capillaries found in the TA muscle. It can be very challenging to study capillary density in intact TA muscles, but capillary density is a measure for which tissue sections are very well-suited. Capillary density may be an important consideration for studies of muscle fatigue.
Intrinsic Tongue of a Mouse Model of Down Syndrome
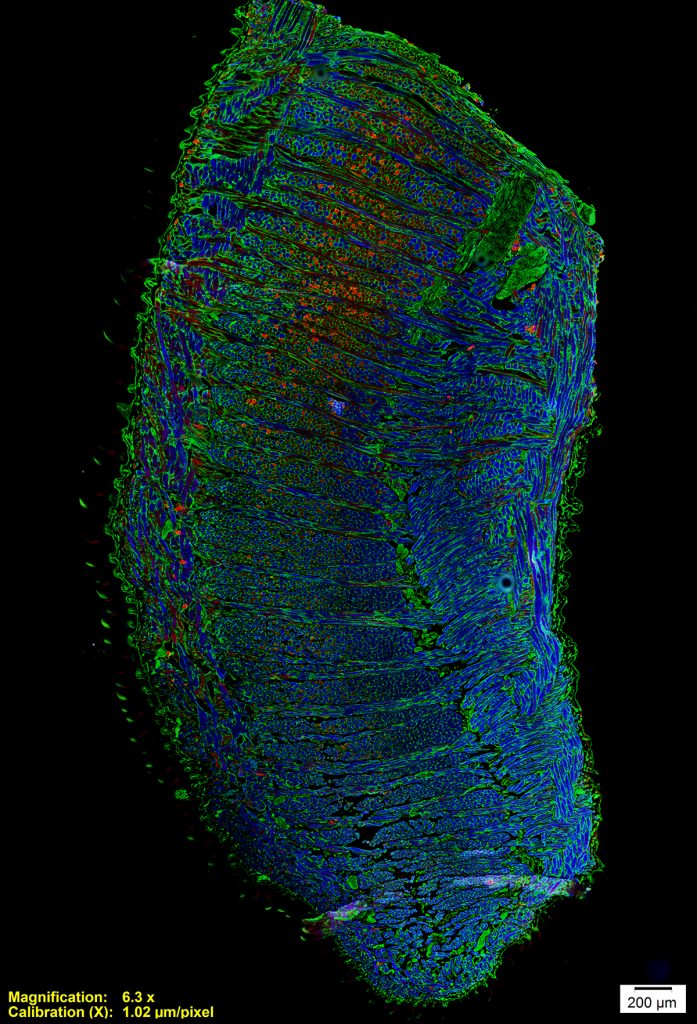
Tiffany Glass, PhD, MA, CMI, Otolaryngology-Head & Neck Surgery
This is a microscopy image of the intrinsic tongue muscles of a mouse model of Down syndrome. This image was taken with an epifluorescence microscope. The dorsal tongue is to the left and the ventral tongue is to the right. Antibody staining shows muscle fiber outlines in green, fast muscle fibers in blue, and relatively slower muscle fibers in red. This image shows us how the intrinsic tongue is made up of complex interlocking of multiple muscles, each with a different fiber orientation, and that different regions of the tongue are composed of different types of muscle fibers. Down syndrome is associated with developmental differences involving tongue function, and tissue studies of mouse models can help us understand these differences.
Mucus – The Vocal Fold Warrior
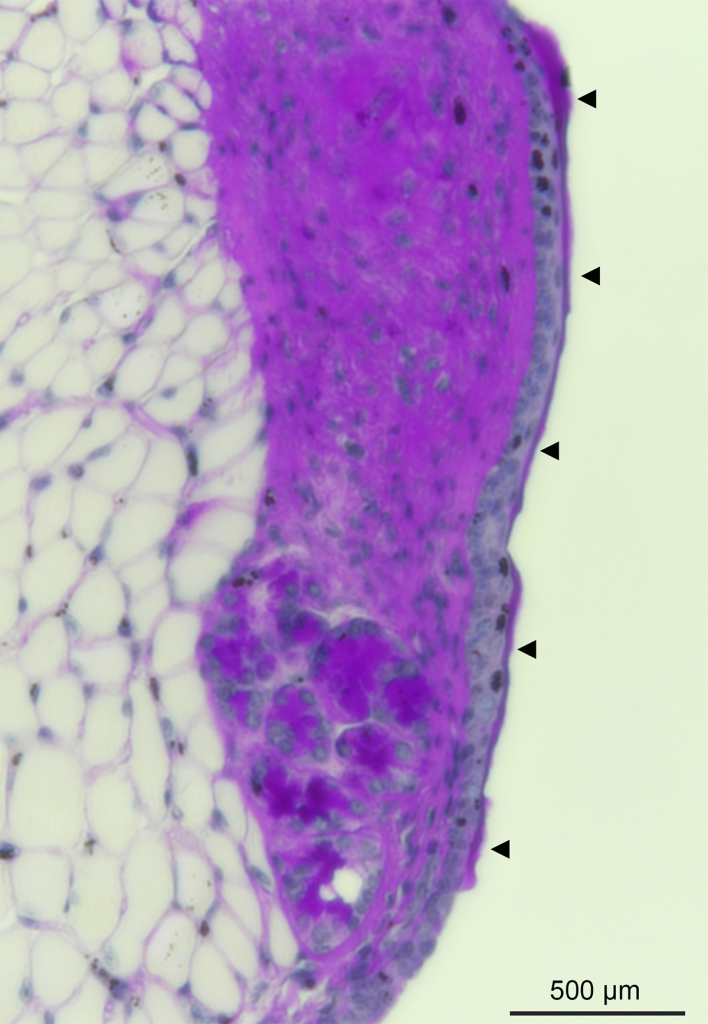
Ran An, PhD, Postdoctoral Fellow, Otolaryngology-Head & Neck Surgery; Susan Thibeault Lab
This is the first depiction of the presence of a thin mucus layer in a mouse model, which covers the vocal fold and serves to support its optimal biomechanical properties and promote voice quality. To get the image, mouse vocal fold tissue was fixed, sectioned, and stained with appropriate histological methods, and subsequently imaged with a Nikon Eclipse Ti2 inverted microscope. The mucus layer forms a viscoelastic physical barrier that protects the underlying tissue from being exposed to external irritants, such as pathogens, particles, and toxic chemicals in inhaled air and gastroesophageal reflux. It is also a natural habitat for laryngeal microbiota, and therefore essential for host-microbe interaction studies in the larynx. Arrows indicate the mucus layer in the image.
Brain Activity in an Adult Rat
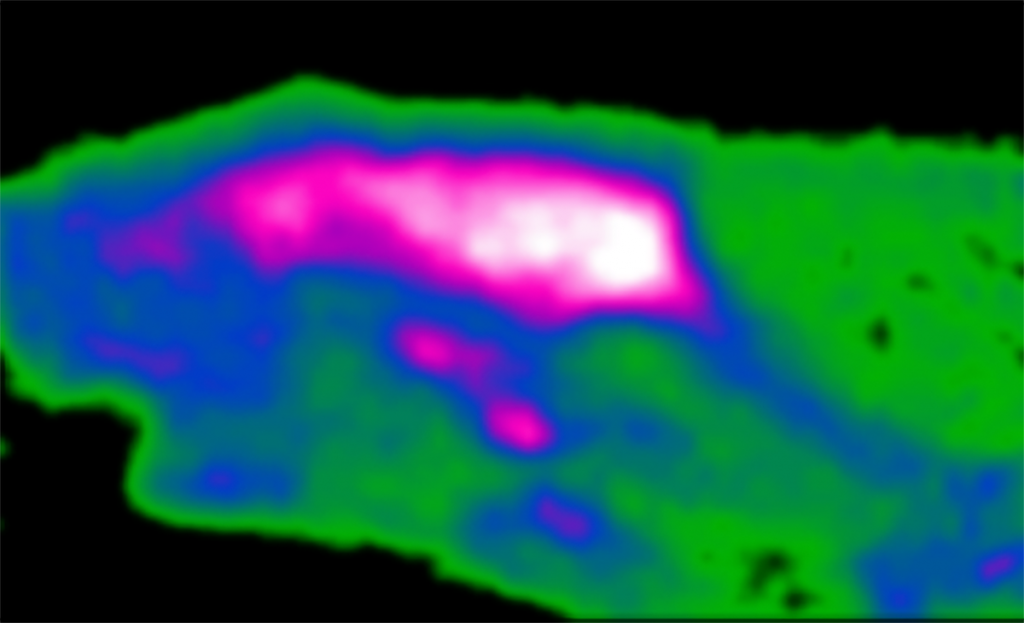
Maxim Slesarev, Academic Staff, Otolaryngology-Head & Neck Surgery (& other non-surgery); Alexander Converse Lab
Maxim Slesarev, neuroimaging technologist, Waisman Center; John Szot, research specialist, Department of Surgery – Otolaryngology; Alex Nisbet, research specialist, Department of Surgery – Otolaryngology; Michelle Ciucci, PhD, professor, Communication Sciences and Disorders, Surgery-Otolaryngology Head & Neck Surgery; Alexander Converse, senior scientist, Waisman Center
This is a cross-section of a rat brain taken with a positron emission tomography scanner. A tracer molecule similar to sugar is tagged with a short-lived radioactive atom and injected through a vein. It then circulates throughout the body and is trapped where sugar is consumed. The colors show brain activity. Those regions with the most activity are colored white, followed by magenta, blue, and green in descending order. These images help us determine how brain activity is different in healthy states vs. neurologic disease so we can develop better treatments. We also use this technique to determine if our treatments are slowing disease progression.
Individuality in the Oropharyngeal Swallow
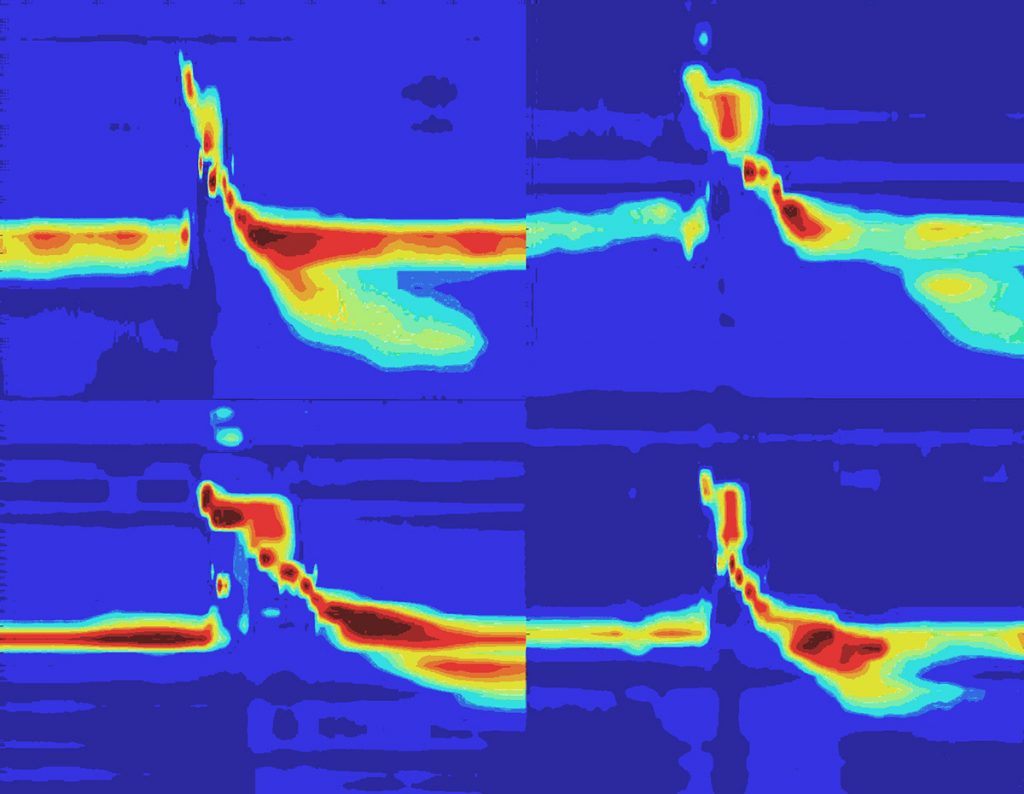
Sophia Colevas, Medical Student, Otolaryngology-Head & Neck Surgery; Timothy McCulloch, MD, Lab
Authors: Sophia M Colevas; Corinne A Jones, PhD; Timothy A McCulloch, MD
High-resolution manometry is a technique in which closely spaced sensors are used to measure the pressure generated over time during the first phase of swallowing (between the mouth and the entry to the airway). This image depicts the swallow pressure profiles of four healthy individuals who were asked to take their normal, comfortable sip of liquid (high pressure shown in red and low pressure shown in blue). At first glance, the swallows appear uniform, but upon closer inspection, the pressure patterns differ between individuals. This reflects the complexity of the initial phase of swallowing where many sensory inputs and multiple muscles must be integrated to complete the swallowing task. A high-resolution measurement tool is necessary to accurately capture this complexity and reach a more complete understanding of human swallowing physiology.